Open Access | Peer-reviewed | Review Article
Nikita Vijay Lakkundi*
Zydus Corporate Park, Scheme No. 63, Survey No. 536, Khoraj (Gandhinagar), Near Vaishnodevi Circle, S. G. Highway, Ahmedabad – 382481, India.
Ruhi Raj
Zydus Corporate Park, Scheme No. 63, Survey No. 536, Khoraj (Gandhinagar), Near Vaishnodevi Circle, S. G. Highway, Ahmedabad – 382481, India.
K.S.Laddha
Institute of Chemical Technology (ICT ), Nathalal Parekh Marg, near Khalsa College, Matunga, Mumbai – 400019, India.
Published: October 24, 2020 DOI: 10.5281/zenodo.4127074
Abstract
Every liquid-solid interface is subject to an interfacial tension also known as surface tension. Agents that reduce this interfacial tension are termed as surfactants. Surfactants in the lungs are associated with two major functions namely reduction of surface tension to prevent the collapse of alveoli post expiration and modulating the lung environment to prevent pathogenic harm. Surfactants help by governing cellular functions since it orchestrates the communication, modification, and trafficking of secretory and membrane proteins which trigger immune pathways. Acute Respiratory Distress Syndrome (ARDS) which is very common in premature babies has been treated extensively in with pulmonary surfactants in the past with a 50% rise in positive outcomes. Although ARDS in COVID-19 is novel, SARS-CoV and SARS-CoV2 both use ACE2 (Angiotensin converting Enzyme) as their host cell receptor to establish infection. ACE2 is expressed in almost all cells in the body. ACE2 is highly expressed on surfactant producing type 2 alveolar cells in the lungs, and on ciliated and goblet cells in the airways; these cells likely provide a portal of entry for the virus in humans. There is currently no pharmaceutical agent to help the critically ill patients except for Positive end expiratory pressure (PEEP) and Non-invasive ventilation (NIV), we should not overlook that the role of surfactant might benefit these kinds of patients by helping improve oxygenation. This review will help understand how an old weapon of surfactant could benefit COVID battle armamentarium and help save lives. After a brief discussion on whether or not surfactant proteins, especially SP-D, should be included; this review also tries to explore the immune-modulation of surfactant protein (SP-D) and connection in COVID disease and its outcomes in obese, diabetic, and co-morbid people. This review emphasizes the dwindling surfactant balance as a putative therapeutic target for the treatment of COVID complications, and therefore, the urgent need for the development of better and safe agents for the severe respiratory syndrome of corona viral disease.
Keywords: Surfactant therapy, Type II cells, pneumocytes, DPPC, biomarker of COVID19, pulmonary; Coronavirus; SARS-CoV-2, Acute Respiratory Distress Syndrome, Surfactant Protein.
| Citation: Nikita Vijay Lakkundi et.al. (2020) Surfactant therapy and SP-D in managing COVID-19 ARDS – therapeutic role possible ?, Journal of PeerScientist 3(2): e1000029. |
| Received: August 27, 2020; Accepted: October 10, 2020; Published: October 24, 2020. |
| Copyright: © 2020 Nikita Vijay Lakkundi et.al. This is an open - access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. |
| Data Availability: All relevant data are within the paper and its Supporting Information files. |
| Funding:This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors, apart from the authors personal resources. |
| Competing interests: The authors have declared that no competing interests exist. |
| *E-mail: nikitavijaylakkundi@gmail.com | Phone: +91-8097736229 |
Introduction
The Severe Acute Respiratory Syndrome Coronavirus -2 (SARS-CoV-2) is a new pathogen that is highly contagious, can spread quickly, and must be considered capable of causing enormous health, economic and societal impacts in any setting [1]. It has affected almost 35 million people and already more than 1.4 million deaths have been reported all over the world [2]. Corona viruses are a group of viruses that were initially hosted only by Bats and Civets but now have spread to humans. A pandemic like the current one had been predicted long ago by researchers working on this group of viruses [1]. The Severe Acute Respiratory Syndrome epidemic in 2009 was also similar to this Coronavirus Disease 19 (COVID-19) epidemic with lesser mortality and milder infection rates [3]. Some patients although have recovered, but significant fatalities have been observed in patients with weak immune systems, pre-existing co-morbid conditions. Those who progress to severe disease, usually report the presence of mild or severe Acute Respiratory Distress Syndrome (ARDS).
ARDS which is interchangeably abbreviated from Acute Respiratory Distress and Adult Respiratory Distress is caused by several causative agents like gastric acid aspiration, thoracic trauma, pneumonia, pathogenic entry and near-drowning [4]. The latter is true for COVID-19 where the Lungs of COVID-19 patients have been compared to that of a person with near drowning experience. ARDS caused due to any of the above reasons in neonates has been successfully treated using surfactant therapy in the past [5-7]. Surfactant replacement therapy (SRT) has been previously tried in ARDS in adults also but with less success [8]. However, we need to gear up existing arms in our armamentarium to fight COVID-19, rectify what went wrong, and gather more data concerning usage of SRT in COVID-19 affected adults who are in critical condition. This review article lays a rational foundation for further research in near future.
ARDS and COVID-19 – Progression and Characteristics
Many reports suggest enough similarities between pneumonia, regular ARDS, and ARDS seen in COVID-19 patients [9-10] compared to mild ARDS patients, those with moderate and severe ARDS had higher mortality rates [11]. Also, SARS-CoV-2 shares 79% sequence identity to SARS-CoV and around 50% to Middle East respiratory syndrome (MERS)-CoV [12]. A study of the largest cohort of >44,000 persons with COVID-19 from China showed that progression in illness severity can range from mild to critical [13] while 81% of this population suffered from only mild to moderate (mild symptoms up to mild pneumonia). The rest of the people i.e. approx. 20% of people suffered from severe (14%) to critical disease (5%). The symptoms in severely ill patients were dyspnea, hypoxia, or >50% lung involvement on imaging.
ARDS frequently starts with hypoxia and respiratory problems which worsens in selected individuals [15-17]. In an observational study conducted in 201 COVID-19 patients of Wuhan, (41.8%) developed ARDS, and of those 84 patients, 44 (52.4%) died. In those who developed ARDS, compared with those who did not, more patients presented with dyspnea (shortness of breath) (50 of 84) [59.5%] patients [18]. Hypoxia (absence of enough oxygen) is another mainstay that presents after average 8-12 days in most patients and suggested by many reports from Wuhan as well as other parts of the world [15-17,19].
Recent studies revealed 50% patients developed hypoxemia after Day 8 of SARS-CoV-2 infection [20]. The actual cause of COVID-19 disease deaths is also important in interpreting case fatality rates, which, is sometimes complicated by shock and multiple organ failure but the real course of the disease is not yet well described [21]. The mortality rate for COVID-19 ARDS ranges from 65.7% to 94% and is largely due to respiratory failure (53%,) , followed by respiratory failure combined with cardiac failure (33%), myocardial damage and circulatory failure (7%), rest death from an unknown cause [22]. These studies might demonstrate the claim that the major morbidity and mortality from COVID-19 is largely due to acute viral pneumonitis that evolves to acute respiratory distress syndrome (ARDS) [23]. These studies might be suggestive that the hypoxic atmosphere in the body can be a trigger to immune activation and sepsis formation that will precede ARDS following multiple organ failure [24-25].
Administration of exogenous pulmonary surfactant might be one such strategy to help revive critically ill patients. Clinical practice guidelines for COVID-19 have suggested measures to reduce the secretion and improve pulmonary ventilation [2]. Thus, SRT might play a role in improving pulmonary ventilation. SARS-CoV infection causes airway resistance and increased EF50 (mid-breath exhalation force), indicating that respiratory function is compromised, and animals must do more work to breathe. A study by Gralinski et al (2015) and Menachery et al (2015) indicate that it is the exhalation portion of each breath that is impacted by SARS-CoV infection, likely due to extensive debris clogging the conducting airways [26]. Also autopsies of COVID-19 lungs resemble that of lungs subject to wet drowning [27-28]. Hence, atleast theoretically, surfactant therapy is promising [27-28].
Therefore, we hypothesize that the SRT of such clogged airways can help critically ill patients. This review will theoretically highlight how surfactants can help in airway clearance. They also manage immune activation, to some extent, if not entirely based on previous research on surfactants and their role in host immune defenses. Additionally, surfactant protein depletion or absence tends to be associated to sever disease outcome. Furthermore, surfactant protein D has been explored for its link to severe COVID 19 outcomes Evidences which link severe disease outcomes to surfactant proteins, especially the role of SP-D have also been discussed in this review article.
Surfactant and ARDS relationship – Have we defined it in COVID-19?
Pulmonary surfactant lines the alveoli and lowers surface tension. It thereby reduces the work associated with breathing. It also prevents atelectasis i.e. lung injury during breathing. A Pulmonary Surfactant consists of a unique phospholipid, termed dipalmitoyl-phosphatidylcholine (DPPC), and four surfactant-associated proteins, Surfactant Protein (SP) / Surfactant Protein -A (SP-A), SP-B, SP-C, and SP-D [29]. DPPC is chemically the ideal surfactant phospholipid, but it lacks the physical properties for lowering surface tension at body temperature (37°C) [26,30]. Hence the addition of surfactant proteins is of importance. Both SP-B and SP-C greatly enhance the adsorption of pulmonary surfactant lamellar bodies [31]. These increase the surfactant production as well as recycling by re-spreading the compressed surfactant film [32]. SP-A is closely involved in film formation of phospholipid mixtures containing SP-B [33]. SP-C and SP-B are involved in biophysical aspects of the maintenance of the surfactant layer in the lungs. SP-B seems to be the most active protein in terms of interfacial behavior; most of the synthetic surfactants have been designed to contain a peptide resembling it. Early in the '90s, a synthetic peptide named as KL4 was developed as a very simple surrogate able to mimic SP-B behavior [34].
Biophysical use of surfactant in COVID ARDS – The relationship
The Gas exchange happens in alveoli which are made of Type II pneumocytes and alveolar macrophages. Type II cells are involved in the production of pulmonary surfactants [34]. COVID-19 causes desquamation of these Type II cells which leads to alveolar dysfunction, edema, and hemorrhage. ARDS patients infected with COVID-19 frequently exhibited reduced surfactant levels [12,35-36]. The decrease of surfactant in the lungs, precisely in alveoli with the increase of the surface tension, the alveoli will tend to collapse. As a result, the whole lung collapses and there is a decrease in its volume as shown in Figure 1. Continuous decreasing lung volume and expanding inward air volume of inspiration will create a decrease in pressure in interstitial space. This decrease in pressure tends to attract, in the interstitial space, liquids, and anti-inflammatory substances, giving rise to interstitial pneumonia. Severe interstitial pneumonia is a late symptom of COVID-19 and the patient is usually intubated. This pressure, exerted in the lung by the air pushed by the pulmonary ventilator during the inspiratory act, prevents small alveoli from collapsing and at best also tends to restretch them. Patients subjected to assisted ventilation might not entirely benefit, since again lack of surfactant will cause a relatively low-pressure area, this continues to draw the interstice liquids and substances that have mostly inflammatory characteristics [37].
Another detailed explanation can be provided by leakage of fibrinogen into alveolus in inflammatory diseases as ARDS, severe pneumonia. Interestingly, leakage of fibrinogen and preceding formation of blood clots in the lungs as well as in the systemic circulation was also seen in COVID-19 (figure 1) [38-39]. This is converted into fibrin due to a pronounced pro-coagulatory activity in the alveolar compartment. Surfactant function is greatly inhibited due to its exhaustion into polymerizing fibrin. The persistence of this ‘specialized’ fibrin matrix promotes fibro-proliferative processes ‘collapse induration’, whereas complete lysis results in the liberation of intact surfactant material with re-opening of formerly collapsed alveoli [38]. Hence a SRT might benefit in COVID ARDS patients.
Neonatal ARDS has been and still is extremely common in clinical settings. The increased survival of neonates, even premature ones, can be attributed to efficient SRT. Initially, Natural porcine or calf lung lavage was being used but advances in technology gave rise to improved artificial surfactant compositions. Extensive reviews on compositions of SRT have been discussed [40]. Notably, these exogenous SRT compositions have been using more of SP-B and SP-C but not SF-A and SF-D. The role of SF-A and SF-D has been associated with immunomodulation [41]. Although SP-A and SP-D do not have a direct function in the surface tension activity of surfactant, these hydrophilic surfactant components play an essential role in innate lung host defense [42] and modify immune responses. However, the exact mechanism by which each of these molecules exert their action is still not known [36].
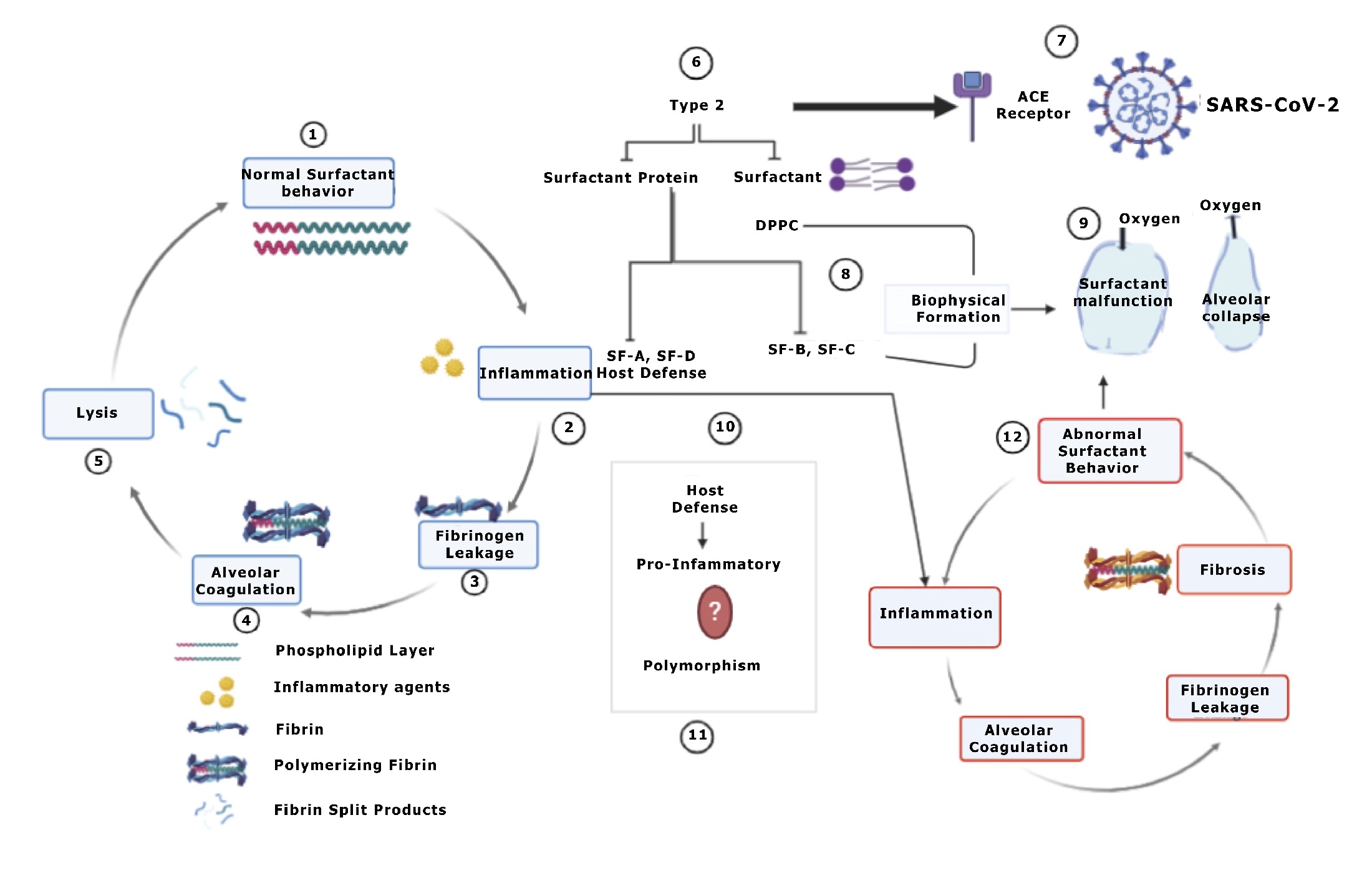
Figure 1: 1-5-Under normal physiological conditions the phospholipid layer at the air–water interface reduces the surface tension and even after inflammatory insult by normal inspired foreign particles, manages to dissociate the fibrotic clots if any. 5-Surfactant then dissociates, and fibrin split products undergo degradation. COVID viral particles attach to ACE receptors on Type 2 cells which are the surfactant producing cells also (6-10). These surfactant and surfactant proteins are involved in biophysical film formation as well as Host defenses. (2) These reduce inflammation but to an extent, yet the role of SF-D and SF-A still is controversial. These hydrophobic Surfactant proteins are also said to be pro-inflammatory in their role and this might be attributed to its polymorphic behavior or difference in the binding sites with different agents. 12-Nonetheless pronounced Inflammation and added abnormal surfactant behavior will lead to alveolar collapse (3) and fibrosis this is due to a pronounced procoagulatory activity in the alveolar compartment (1). The surfactant function is greatly inhibited by the incorporation of hydrophobic surfactant components (PL, SP-B/C) into polymerizing fibrin.
What is the role of SP-D?
Pulmonary SP-D is a defense lectin promoting clearance of viral infections [35]. SP-D is recognized to bind the S protein of SARS-CoV and enhance phagocytosis [43-44]. Non-controlled case studies suggest that virus-induced pneumonia indeed can be efficiently treated with instillation of surfactant preparations that contain SP-D [43-44]. SP-D binds to viral bodies and triggers immune responses. Another role of SP-D is the stabilization of pathogen and removal of inflammatory molecules inhaled into airway secretion. SP-D also contributes to the local differentiation of freshly recruited monocytes in alveolar cells. It inhibits bacterial clearance via enhancing phagocytosis and direct bactericidal effect. Numerous studies have shown that, under pathological conditions, SP-A and SP-D can each undergo a variety of post-translational modifications (such as oxidation, nitration, S-nitrosylation) that can lead to loss of function [45-46].
Role of SP-D in usual ARDS and COVID-19 ARDS: Should be or should not be?
It is still unclear as to whether there is complete depletion of SP-D or loss of function of SP-D in COVID-19. The latter is more probable as per a recent study [44-45] which reveals elevated SP-D levels in the patients suffering from critical ARDS. Notable is the fact that COVID-19 ARDS differs from normal or idiopathic ARDS which showed reduced SP-D levels. Contrary to this recent studies for neonatal ARDS reported an increase in SP-D level. In preclinical models, SP-D deficient mice were found with significantly increased surfactant lipid molecules in their Bronchoalveolar Lavage (BAL) [47] and that might conversely; reiterate to some extent that increased SP-D predicts surfactant depletion should not be ignored in COVID-19. In ARDS patients, surfactant therapy might be of some benefit if not a complete cure.
Discussion
The lung collectins have capacity to recognize carbohydrates, lipids, and proteins found in the surface of pathogens [35-36] and on the other hand, it binds and modulates the activity of receptors on immune cells, this might explain their role in modulating lung immunity [48]. It is said that lipid components of surfactant appear to have a predominantly immunosuppressive effect, and this has been seen in the synthetic surfactant, Adsurf and Exosurf. In contrast, the surfactant-associated proteins have been reported to exhibit both pro-inflammatory and anti-inflammatory activity as seen with Curosurf, Bovine Lipid Extract Surfactant (BLES) and alveofact [49-50].
A good number of clinical trials of exogenous surfactant as a potential therapy to treat ARDS patients had failed. This failure can be attributed to surfactants inactivation. Therefore, it was assumed that treating ARDS patients via surfactant replacement therapy cannot be considered because of the lack of benefits. Surfactant preparations derived from animals are considered to be the most effective for improving respiratory function. However, these therapeutic materials have important limitations:
- A risk of pathogen transmission with substantial compositional variability between batches,
- They lack collectin surfactant proteins (SP-A and SP-D) and in most cases contain a reduced amount of hydrophobic proteins (SP-B and SP-C) compared with the endogenous surfactant,
- Important properties like the viscosity of surfactant suspension, or the fluidity of the surface film can be affected by the lipid composition as it is modified compared with that of endogenous surfactant and,
- The expensiveness of animal-derived surfactants, particularly considering the large dosage required to treat adult ARDS patients. Many groups of scientists are trying to understand the underlying molecular mechanism of surfactant inhibition caused by agents reaching the airways as a consequence of ARDS, as a basis for the development of new synthetic exogenous surfactants with enhanced resistance to inhibition and producible in sufficient amounts to treat adult patients [28,40].
A study has already highlighted the different surfactant characteristics and their clinical evidence outcomes [51]. The medical fraternity has slowly started pondering whether this kind of therapy will be useful in treating ARDS COVID 19 patients. Many clinical trials are on-going for use of surfactants in COVID-19 patients as enlisted in Table 1. The first study [52] was a Retrospective analysis of patients who received off-label use of natural surfactant during the COVID-19 pandemic. Seven COVID-19 PCR positive ARDS patients received liquid Curosurf (720 mg) in 150 ml normal saline, divided into five 30 ml aliquots) and delivered via a bronchoscope into second-generation bronchi. Patients were matched with 14 comparable subjects receiving supportive care for ARDS during the same time period. A 28-days mortality reduction was achieved in the surfactant group, results were not significant. Another Study from Italy [53] involved Surfactant administration in five critically ill patients. Surfactant (Curosurf) was instilled at the dosage of approximately 30 mg/kg of lean body weight (LBW) 7 diluted with normal saline (2 ml/kg LBW). Respiratory parameters were recorded before administration (T0) and 6 (T1), 12 (T2), 18 (T3), 24 (T4), 36 (T5) and 48 (T6) h apart. Physiological outcomes (change in PaO2/FiO2 and COVID-19 patients with very severe hypoxia and low pulmonary compliance were treated with intra-tracheal natural surfactant. All patients were found to have a physiological improvement and there was a positive outcome in four. One patient died but the reason of death was not SRT. Although this is a small cohort, Clinicians reported 80% of 30-day survival rate despite the severity of the patients. Many clinical trials are on-going for use of surfactants in COVID-19 patients. The same has been enlisted in table 1 and we hope there’s a light at the end of the tunnel. As far as existing clinical trials are concerned on the role of Surfactant in Adult ARDS, controversy still exists.
A meta-analysis published by Meng et. Al. (2018) stated that SRT has no evidence in preventing mortality. Statistically insignificant but nonetheless positive outcome was achieved in the oxygenation status of treated patients [54]. Notably, this meta-analysis also highlights several limitations, which include small sample size, the unequivocal blinding method, the inclusion of severely ill patients, etc. Individual trial failure might also be because of an insufficient dosage of surfactant and faulty route of delivery [31]. Surfactant inhibition occurring due to accumulated edema proteins was published in a report by Gewolb et. al. (2020). This has not been considered in many clinical trials [45].
Surfactant, SP-D, Gender and Co-morbidities – Is there a link?
The role of surfactant and surfactant proteins is thoroughly being researched and its role in co-morbid condition patients of COVID-19 namely obesity, Type 2 Diabetes Mellitus (T2DM), and Cardiovascular Disease (CVD) cannot be ignored. Patients who are men, smokers, or suffering from T2DM/Obesity and CVD are highly susceptible to have the worst prognosis when affected with COVID-19 [56-57]. Interestingly these conditions also affect the worst outcomes in ARDS irrespective of the cause of ARDS. This makes above susceptible population the main target if SRT is to be initiated and we believe existing knowledge about the role of surfactant and surfactant proteins in these associated conditions will help tackle the problem. Changes in SP-D and Lung surfactant production in various Co-morbid conditions have been summarized in Table 2. On the contrary, both, presence of obesity and T2DM revealed increased SP-D circulation in group of patients subject to endurance exercise training decreased serum levels of surfactant protein D and improved aerobic fitness [61].
SP-D should be really included?
A group of researchers is developing recombinant SP-D protein for its use in surfactant therapy in COVID-19 patients. The role of surfactant proteins especially SP-D is interesting because of the dual role that SP-D plays during lung diseases. Recent trials suggest mixed results in quantities of circulating SP-D seen in conditions like viral pneumonia [62]. A recent study also suggests that level of SP-D in COVID-19 patients were elevated, prolonged elevation was particularly noted in the patients of worse prognosis [42,63]. This might lead to the conclusion that SP-D might be a biomarker and help detect the worst outcome patients. Researchers should be aware that SP-D polymorphisms tend to misbalance the pro-inflammatory /anti-inflammatory ratio and supposedly anti-inflammatory SP-D might be more risky than beneficial [64-65].
Table 1: Brief details of current ongoing trials using Surfactant Therapy: 

Table 2: Changes in SP-D and Lung surfactant production in various Co-morbid conditions: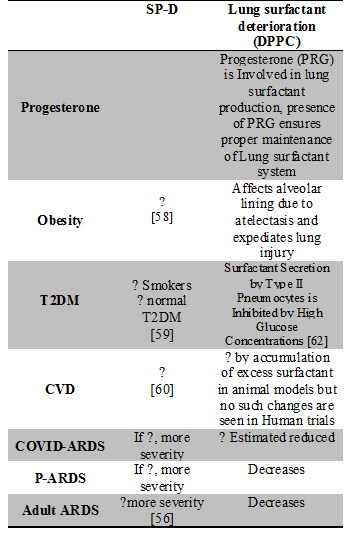
 Contrarily we cannot rule out the cause of elevated SP-D in COVID-19 patients, which may also be a result of secondary infections in COVID-19 presenting ARDS. Whether or not coronavirus increases SP-D yet remains unsolved but SRT remains a considerable choice given its role in cytokine suppression [50]. The ultimate cytokine storm of COVID-19 can be tackled with an existing tool and help save a precious life.
Contrarily we cannot rule out the cause of elevated SP-D in COVID-19 patients, which may also be a result of secondary infections in COVID-19 presenting ARDS. Whether or not coronavirus increases SP-D yet remains unsolved but SRT remains a considerable choice given its role in cytokine suppression [50]. The ultimate cytokine storm of COVID-19 can be tackled with an existing tool and help save a precious life.
Conclusion
The majority of the COVID-19 deaths are being attributed to sudden shortness of breathing, cardiac fatality and pneumonia with or without ARDS. Interestingly, studies have shown the latter being frequently observed. The major symptom for COVID-19 has shown ground glass appearance on chest radiography, suggesting hypoxia. The literature review of this topic shows that it can be a beneficial option to save critical patients who are subjected to ventilators. Surfactant therapy can also prevent the harm caused by mechanical ventilators and should be now considered as a better adjuvant therapy for treating critically ill patients. The role of SP-D should or shouldn’t be included, remains a question mark. As recent investigations on elevated serum levels of SP-D has showed negative co-relation to disease outcome. However, we still cannot rule out the usage of surfactant as advantageous since recent observational studies have a positive outcome in treating critically ill patients.
A safe vaccination drug is still many months away. The latest findings are also indicating re-infections in many patients. Not to forget there are many neurological as well as psychological concerns that are being discovered during and after the infection. The world right now is overwhelmed with the intensity of changes brought to society due to this pandemic. Our review highlights how clinicians can consider using surfactant therapy at least as a compassionate use in severely ill patients. All we need to do is a strategic tweak in the SRT composition with proper addition or deletion of recombinant SP-D in particular sets of patients to receive better outcomes. Further research and large clinical trials are still necessary yet SRT even now remains a lucrative option in increasing oxygenation and saving critically ill patients.
Authors’ contribution: All the authors mentioned have equal contribution in designing, data collection, data analysis, drafting, finalizing and approving the manuscript.
References
- Sharma, Atul, et al. "Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2): A global pandemic and treatments strategies." International Journal of Antimicrobial Agents (2020): 106054.
- https://www.who.int/docs/default-source/coronaviruse/situation-reports (Accessed on 28th September 2020)
- Drugs & Diseases> Medscape > Clinical Practice Guidelines Rapid Advice for COVID-19 Clinical Practice Guidelines (2020) Available online at https://reference.medscape.com/viewarticle/927431 (Accessed on 28th September 2020)
- Peck, Kayla M., et al. "Coronavirus host range expansion and Middle East respiratory syndrome coronavirus emergence: biochemical mechanisms and evolutionary perspectives." Annual review of virology 2 (2015): 95-117.
- Robertson, Bengt, and Henry L. Halliday. "Principles of surfactant replacement." Biochimica et Biophysica Acta (BBA)-Molecular Basis of Disease2-3 (1998): 346-361.
- Hentschel, Roland, et al. "Surfactant replacement therapy: From biological basis to current clinical practice." Pediatric Research (2020): 1.
- Amigoni, Angela, et al. "Surfactants in acute respiratory distress syndrome in infants and children: past, present and future." Clinical drug investigation8 (2017): 729-736.
- Shi, Heshui, et al. "Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study." The Lancet Infectious Diseases (2020).
- Raghavendran, Krishnan, D. Willson, and R. H. Notter. "Surfactant therapy for acute lung injury and acute respiratory distress syndrome." Critical care clinics3 (2011): 525-559.
- Wu, Zunyou, and Jennifer M. McGoogan. "Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention." Jama13 (2020): 1239-1242.
- Zhao, Dahai, et al. "A comparative study on the clinical features of COVID-19 pneumonia to other pneumonias." Clinical Infectious Diseases (2020)
- Liu, Yanli, et al. "Clinical features and progression of acute respiratory distress syndrome in coronavirus disease 2019." MedRxiv (2020).
- Wang, Yixuan, et al. "Unique epidemiological and clinical features of the emerging 2019 novel coronavirus pneumonia (COVID‐19) implicate special control measures." Journal of medical virology6 (2020): 568-576.
- Phua, Jason, et al. "Intensive care management of coronavirus disease 2019 (COVID-19): challenges and recommendations." The Lancet Respiratory Medicine (2020).
- Yi, Ye, et al. "COVID-19: what has been learned and to be learned about the novel coronavirus disease." International journal of biological sciences10 (2020): 1753.
- Schaible, Bettina, Kirsten Schaffer, and Cormac T. Taylor. "Hypoxia, innate immunity and infection in the lung." Respiratory physiology & neurobiology3 (2010): 235-243.
- Jin, Ying-Hui, et al. "A rapid advice guideline for the diagnosis and treatment of 2019 novel coronavirus (2019-nCoV) infected pneumonia (standard version)." Military Medical Research1 (2020): 4.
- Li, Xu, and Xiaochun Ma. "Acute respiratory failure in COVID-19: is it “typical” ARDS?." Critical Care 24 (2020): 1-5.
- Bhatraju, Pavan K., et al. "COVID-19 in critically ill patients in the Seattle region—case series." New England Journal of Medicine21 (2020): 2012-2022.
- Yang, Xiaobo, et al. "Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study." The Lancet Respiratory Medicine (2020).
- Wu, Chaomin, et al. "Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China." JAMA internal medicine (2020).
- Huang, Chaolin, et al. "Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China." The lancet10223 (2020): 497-506.
- Auwaerter, Paul G. "Coronavirus COVID-19 (SARS-2-CoV)." Johns Hopkins ABX Guide (2020).
- Vincent, Jean-Louis, and Fabio S. Taccone. "Understanding pathways to death in patients with COVID-19." The Lancet Respiratory Medicine5 (2020): 430-432.
- Chen, Jun, and Kanta Subbarao. "The immunobiology of SARS." Rev. Immunol. 25 (2007): 443-472.
- Chen, Tao, et al. "Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study." Bmj 368 (2020).
- Gralinski, Lisa E., and Ralph S. Baric. "Molecular pathology of emerging coronavirus infections." The Journal of pathology2 (2015): 185-195.
- Staudinger, Thomas, et al. "Exogenous surfactant therapy in a patient with adult respiratory distress syndrome after near drowning." Resuscitation2 (1997): 179-182.
- Echaide, Mercedes, et al. "Restoring pulmonary surfactant membranes and films at the respiratory surface." Biochimica et Biophysica Acta (BBA)-Biomembranes9 (2017): 1725-1739.
- Cevik, Muge, Connor Bamford, and Antonia Ho. "COVID-19 pandemic–A focused review for clinicians." Clinical Microbiology and Infection (2020).
- Han, SeungHye, and Rama K. Mallampalli. "The role of surfactant in lung disease and host defense against pulmonary infections." Annals of the American Thoracic Society5 (2015): 765-774.
- Willson, Douglas F., and Robert H. Notter. "The future of exogenous surfactant therapy." (2011): 1369-1388.
- Chakraborty, Mallinath, and Sailesh Kotecha. "Pulmonary surfactant in newborn infants and children." Breathe6 (2013): 476-488.
- Hobi, Nina, et al. "A small key unlocks a heavy door: The essential function of the small hydrophobic proteins SP-B and SP-C to trigger adsorption of pulmonary surfactant lamellar bodies." Biochimica et Biophysica Acta (BBA)-Molecular Cell Research8 (2016): 2124-2134.
- Wheeler, Derek S., Hector R. Wong, and Thomas P. Shanley, eds. The respiratory tract in pediatric critical illness and injury. Springer Science & Business Media, 2008.
- Mulugeta, Surafel, Shin-Ichi Nureki, and Michael F. Beers. "Lost after translation: insights from pulmonary surfactant for understanding the role of alveolar epithelial dysfunction and cellular quality control in fibrotic lung disease." American Journal of Physiology-Lung Cellular and Molecular Physiology6 (2015): L507-L525.
- Khubchandani, Kavita R., and Jeanne M. Snyder. "Surfactant protein A (SP‐A): the alveolus and beyond." The FASEB Journal1 (2001): 59-69.
- Mason, Robert J. "Pathogenesis of COVID-19 from a cell biology perspective." (2020).
- Matthay, Michael A., and Rachel L. Zemans. "The acute respiratory distress syndrome: pathogenesis and treatment." Annual Review of Pathology: Mechanisms of Disease 6 (2011): 147-163.
- Günther, Andreas, et al. "Surfactant alteration and replacement in acute respiratory distress syndrome." Respiratory research6 (2001): 353.
- Mirastschijski, Ursula, Rolf Dembinski, and Kathrin Maedler. "Lung Surfactant for Pulmonary Barrier Restoration in Patients With COVID-19 Pneumonia." Frontiers in Medicine 7 (2020): 254.
- McCormack, Frank. "The structure and function of surfactant protein-A." Chest6 (1997): 114S-119S.
- Lai, Yurong. Transporters in drug discovery and development: detailed concepts and best practice. Woodhead Publishing, (2014).
- Wu, Y. P., et al. "Elevated Plasma Surfactant Protein D (SP‐D) Levels and a Direct Correlation with Anti‐severe Acute Respiratory Syndrome Coronavirus‐specific IgG Antibody in SARS Patients." Scandinavian journal of immunology6 (2009): 508-515.
- Saito, Atsushi, et al. "Serum surfactant protein A and D may be novel biomarkers of COVID-19 pneumonia severity." (2020).
- Watson, Alastair, et al. "Surfactant proteins A and D: trimerized innate immunity proteins with an affinity for viral fusion proteins." Journal of innate immunity1 (2019): 13-28.
- Korfhagen, Thomas R., et al. "Surfactant protein-D regulates surfactant phospholipid homeostasis in vivo." Journal of Biological Chemistry43 (1998): 28438-28443.
- Garcia-Verdugo, Ignacio, et al. "Lung protease/anti-protease network and modulation of mucus production and surfactant activity." Biochimie11 (2010): 1608-1617.
- Mittal, Neha, and Sankar Nath Sanyal. "Cycloxygenase inhibition enhances the effects of surfactant therapy in endotoxin-induced rat model of ARDS." Inflammation2 (2011): 92-98.
- Mittal, Neha, and Sankar Nath Sanyal. "In vivo effect of surfactant on inflammatory cytokines during endotoxin-induced lung injury in rodents." Journal of immunotoxicology4 (2011): 274-283.
- El-Gendy, Nashwa, et al. "Delivery and performance of surfactant replacement therapies to treat pulmonary disorders." Therapeutic delivery8 (2013): 951-980.
- Piva, Simone, et al. "Surfactant Therapy for COVID-19 Related ARDS: A Retrospective Case-Control Pilot Study." (2020).
- Busani, Stefano, et al. "Surfactant replacement might help recovery of low-compliance lung in severe COVID-19 pneumonia." Therapeutic advances in respiratory disease 14 (2020): 1753466620951043.
- Davidson, Warren J., et al. "Exogenous pulmonary surfactant for the treatment of adult patients with acute respiratory distress syndrome: results of a meta-analysis." Critical Care2 (2006): R41.
- Gewolb, Ira H., and Janet O'brien. "Surfactant secretion by type II pneumocytes is inhibited by high glucose concentrations." Experimental lung research3 (1997): 245-255.
- Eisner, M. D., et al. "Plasma surfactant protein levels and clinical outcomes in patients with acute lung injury." Thorax11 (2003): 983-988.
- https://www.hopkinsguides.com/hopkins/view/Johns_Hopkins_ABX_Guide/540747/all/Coronavirus_COVID_19__SARS_CoV (Accessed on 28th September 2020)
- Moreno-Navarrete, José María, and José Manuel Fernández-Real. "The possible role of antimicrobial proteins in obesity-associated immunologic alterations." Expert Review of Clinical Immunology7 (2014): 855-866.
- Fernández-Real, José Manuel, et al. "Surfactant protein d, a marker of lung innate immunity, is positively associated with insulin sensitivity." Diabetes care4 (2010): 847-853.
- Hirano, Yuki, et al. "Surfactant protein-D deficiency suppresses systemic inflammation and reduces atherosclerosis in ApoE knockout mice." Cardiovascular Research10 (2017): 1208-1218.
- Rezaei, Sajjad, et al. "Endurance exercise training decreased serum levels of surfactant protein D and improved aerobic fitness of obese women with type-2 diabetes." Diabetology & metabolic syndrome1 (2017): 1-8.
- Leth-Larsen, Rikke, et al. "Surfactant protein D (SP-D) serum levels in patients with community-acquired pneumonia." Clinical immunology1 (2003): 29-37.
- Kerget, Buğra, et al. "Are Serum Interleukin 6 and Surfactant Protein D Levels Associated with the Clinical Course of COVID-19?." Lung(2020): 1-8.
- Kreitmann, Louis, et al. "Early bacterial co-infection in ARDS related to COVID-19." Intensive care medicine(2020): 1-3.
- Jounblat, R., et al. "The role of surfactant protein D in the colonisation of the respiratory tract and onset of bacteraemia during pneumococcal pneumonia." Respiratory research 6.1 (2005): 126.